Our leading
AI-driven drug discovery pipeline
Leveraging the power of the Recursion OS, we are building an industry-leading pipeline of potential best-in-class and first-in-class therapeutic assets.
We focus on indications with high unmet need, including oncology and rare disease.
>50K*
REC-4881 is an orally bioavailable, non-ATP-competitive, allosteric small molecule inhibitor of MEK1 and MEK2 being developed to reduce polyp burden and progression to adenocarcinoma in people living with FAP, a rare tumor predisposition syndrome. There are currently no FDA-approved therapies for the treatment of FAP. MEK1/2 inhibition to target FAP was identified using phenotypic insights by the Recursion OS.
Learn more on clinicaltrials.gov.
~150,000*
REC-617 is a reversible, non-covalent small molecule CDK7 inhibitor being developed for the treatment of multiple advanced solid tumor indications. There are currently no CDK7 inhibitors approved by the FDA. The precision design of REC-617, resulting in high selectivity and optimized half-life, could distinguish it from other CDK7 inhibitors in development, by enabling the management of potential toxicities associated with CDK7 inhibition and maximizing on-target efficacy.
Learn more on clinicaltrials.gov.
>100,000*
REC-1245 is a highly potent RBM39 degrader being developed for the treatment of biomarker-enriched solid tumor indications and lymphoma. RBM39 was identified using phenotypic insights by the Recursion OS, and is a novel target mimicking CDK12 loss. There are currently no RBM39 degraders approved by the FDA. Early preclinical data shows REC-1245 reduces viability in tumors characterized by replication stress and DNA repair vulnerabilities (DDR defects) across multiple solid tumor types, including MSI-H/dMMR, HRR altered cancers, and other tumors.
Learn more on clinicaltrials.gov
~41,000*
REC-3565 is a small molecule MALT1 inhibitor, being developed for multiple hematology indications. There are currently no MALT1 inhibitors approved by the FDA. The precision design of REC-3565 has resulted in a well-balanced molecule with selectivity over UGT1A1, which distinguishes it from other MALT1 inhibitors in clinical development. Avoiding UGT1A1 inhibition can potentially reduce hyperbilirubinemia risk and allow a better combination profile with drugs that have known liver toxicity issues.
Learn more on clinicaltrials.gov.
~45,000*
REC-4539 is the first LSD1 inhibitor designed to be both CNS-penetrant and reversible, and is being developed for multiple hematology and solid tumor indications, including small-cell lung cancer (SCLC) and acute myeloid leukemia (AML). There are currently no LSD1 inhibitors approved by the FDA. REC-4539’s combined properties distinguish it from other LSD1 inhibitors in development, with the potential to reduce adverse events seen from on-target platelet effects.
>21,000*
REC-7735 is a PI3Kα H1047R mutant selective inhibitor being developed for the treatment of solid tumors including HER2- HR+ breast cancer where the PI3Kα-H1047R mutation is present. This highly selective inhibitor targets the most frequent PIK3CA mutation and a key driver of cancer. REC-7735 was designed to maximize the therapeutic window and to avoid dose-limiting hyperglycemia or other off-target AEs associated with wild-type PI3Kα inhibition. Preclinical models have shown tumor regression when used as a monotherapy treatment and when combined with standard of care.
>7,800*
REC-102 is an orally available, small molecule ENPP1 inhibitor, being developed for the treatment of hypophosphatasia (HPP). HPP is a rare, potentially life-threatening genetic disease, characterized by impaired mineralization of bones and teeth. Inhibiting ENPP1 reduces inorganic pyrophosphate (PPi) levels which may restore the PPi and phosphate balance needed to promote bone mineralization. With our collaborators, we have shown that ENPP1 inhibition is safe and well-tolerated by preclinical models, and for the first time demonstrated that ENPP1 is a druggable target for later-onset HPP.
We partner with leading data & capability partners, and therapeutic partners to discover and develop new medicines across oncology, rare disease, neuroscience, immunology and inflammation. More on our partnerships →
.png)
- 5 program milestones achieved to date
- Joint portfolio of differentiated molecules for challenging targets
- Potential for later stage discovery milestones over next 12-18 months
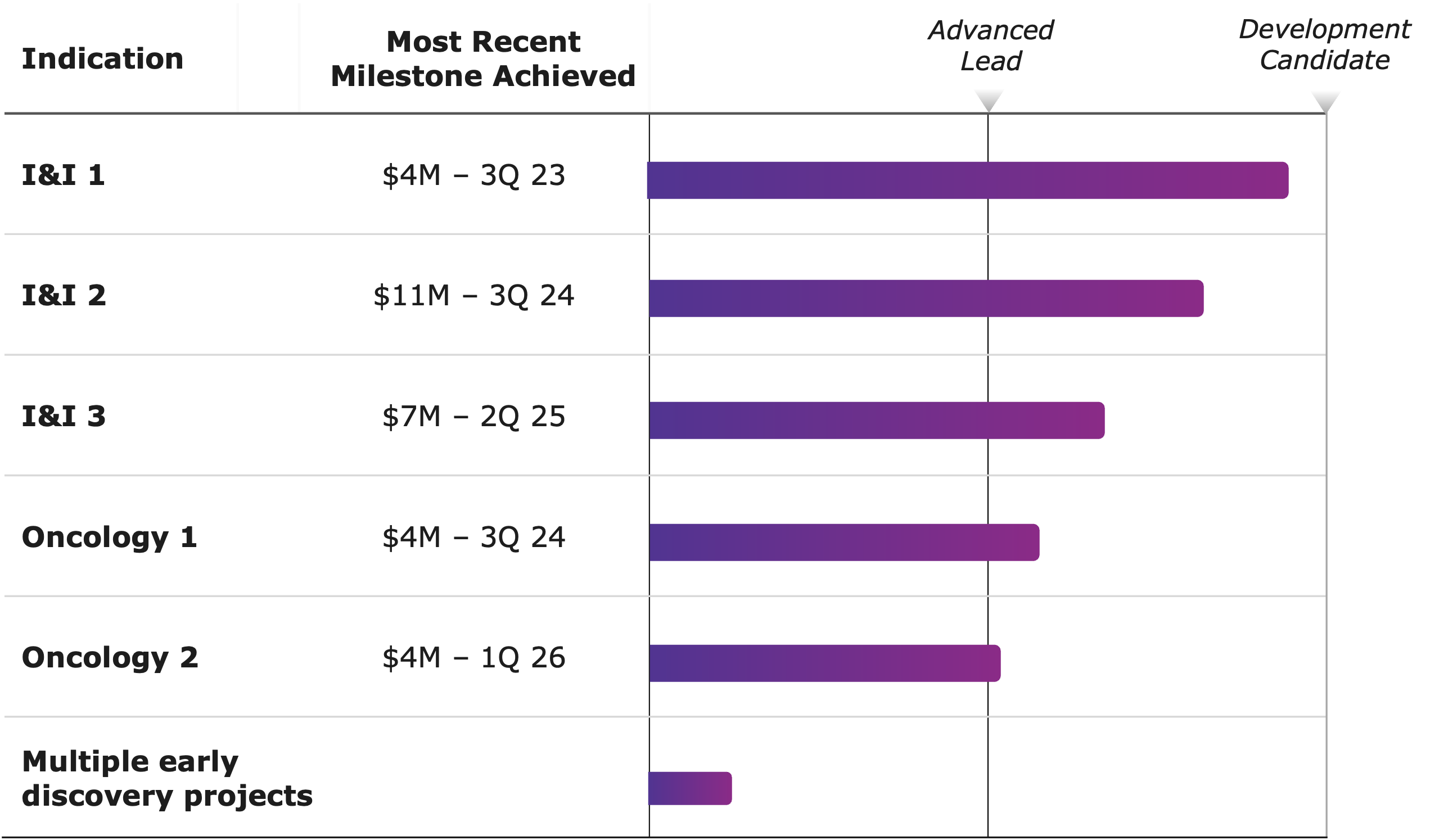

- 6 Phenomaps: 4 GI oncology, 2 neuroscience
- 1 program initiated in GI onc indication

- Advancing programs to lead series milestones
.png)
- Identify and advance first-in-class and best-in-class programs
Leveraging the power of the Recursion OS, we are building an industry-leading pipeline of potential best-in-class and first-in-class therapeutic assets.
We focus on indications with high unmet need, including oncology and rare disease.
MEK Inhibitor
Phase 2
>50K*
REC-4881 is an orally bioavailable, non-ATP-competitive, allosteric small molecule inhibitor of MEK1 and MEK2 being developed to reduce polyp burden and progression to adenocarcinoma in people living with FAP, a rare tumor predisposition syndrome. There are currently no FDA-approved therapies for the treatment of FAP. MEK1/2 inhibition to target FAP was identified using phenotypic insights by the Recursion OS.
Learn more on clinicaltrials.gov.
CDK7
Phase 1/2
~150,000*
REC-617 is a reversible, non-covalent small molecule CDK7 inhibitor being developed for the treatment of multiple advanced solid tumor indications. There are currently no CDK7 inhibitors approved by the FDA. The precision design of REC-617, resulting in high selectivity and optimized half-life, could distinguish it from other CDK7 inhibitors in development, by enabling the management of potential toxicities associated with CDK7 inhibition and maximizing on-target efficacy.
Learn more on clinicaltrials.gov.
RBM39
Phase 1
>100,000*
REC-1245 is a highly potent RBM39 degrader being developed for the treatment of biomarker-enriched solid tumor indications and lymphoma. RBM39 was identified using phenotypic insights by the Recursion OS, and is a novel target mimicking CDK12 loss. There are currently no RBM39 degraders approved by the FDA. Early preclinical data shows REC-1245 reduces viability in tumors characterized by replication stress and DNA repair vulnerabilities (DDR defects) across multiple solid tumor types, including MSI-H/dMMR, HRR altered cancers, and other tumors.
Learn more on clinicaltrials.gov
MALT1
Phase 1
~41,000*
REC-3565 is a small molecule MALT1 inhibitor, being developed for multiple hematology indications. There are currently no MALT1 inhibitors approved by the FDA. The precision design of REC-3565 has resulted in a well-balanced molecule with selectivity over UGT1A1, which distinguishes it from other MALT1 inhibitors in clinical development. Avoiding UGT1A1 inhibition can potentially reduce hyperbilirubinemia risk and allow a better combination profile with drugs that have known liver toxicity issues.
Learn more on clinicaltrials.gov.
LSD1
Phase 1
~45,000*
REC-4539 is the first LSD1 inhibitor designed to be both CNS-penetrant and reversible, and is being developed for multiple hematology and solid tumor indications, including small-cell lung cancer (SCLC) and acute myeloid leukemia (AML). There are currently no LSD1 inhibitors approved by the FDA. REC-4539’s combined properties distinguish it from other LSD1 inhibitors in development, with the potential to reduce adverse events seen from on-target platelet effects.
PI3Kα H1047R
Candidate profiling
>21,000*
REC-7735 is a PI3Kα H1047R mutant selective inhibitor being developed for the treatment of solid tumors including HER2- HR+ breast cancer where the PI3Kα-H1047R mutation is present. This highly selective inhibitor targets the most frequent PIK3CA mutation and a key driver of cancer. REC-7735 was designed to maximize the therapeutic window and to avoid dose-limiting hyperglycemia or other off-target AEs associated with wild-type PI3Kα inhibition. Preclinical models have shown tumor regression when used as a monotherapy treatment and when combined with standard of care.
ENPP1
Candidate profiling
>7,800*
REC-102 is an orally available, small molecule ENPP1 inhibitor, being developed for the treatment of hypophosphatasia (HPP). HPP is a rare, potentially life-threatening genetic disease, characterized by impaired mineralization of bones and teeth. Inhibiting ENPP1 reduces inorganic pyrophosphate (PPi) levels which may restore the PPi and phosphate balance needed to promote bone mineralization. With our collaborators, we have shown that ENPP1 inhibition is safe and well-tolerated by preclinical models, and for the first time demonstrated that ENPP1 is a druggable target for later-onset HPP.
We partner with leading data & capability partners, and therapeutic partners to discover and develop new medicines across oncology, rare disease, neuroscience, and immunology & inflammation. More on our partnerships →
.png)
- 5 program milestones achieved to date
- Joint portfolio of differentiated molecules for challenging targets
- Potential for later stage discovery milestones over next 12-18 months

- 6 Phenomaps: 4 GI oncology, 2 neuroscience
- 1 program initiated in GI onc indication

- Advancing programs to lead series milestones
.png)
- Identify and advance first-in-class and best-in-class programs


